ICH Q3A Guideline Impurities in new drug substance
Duration : 00:00:00 - Like :
Youtube : Download
Description :
...
Related Videos :
 |
Regulatory Considerations for Impurity Qualification: ICH Q3A/Q3C/Q3D, RLD & MDD By: U.S. Food and Drug Administration |
 |
ICH Q3A(R2) Guideline Explained | Impurities in New Drug Substances for Regulatory Compliance By: MedPharma Global Insights |
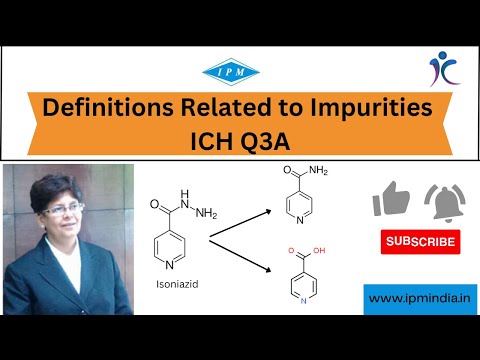 |
Definitions related to Impurities [ICH Q3A] - Institute of Pharmaceutical Management By: Institute of Pharmaceutical Management |
 |
Identified Impurity,Unidentified Impurity, Specified Impurity , Unspecified Impurity as per ICH Q3A By: Pharma Pill |
 |
ICH Q3A l Impurities in New Drug substance l impurities in pharma industry l Question and answers By: PharmGrow |
 |
ICH Q3A Guideline - Impurities in new drug substance By: Regulatory Research Centre |
 |
ICH Q3A & ICH Q3B II Specification of Impurities II Pharma guidelines II Rishabh II Interview By: Rishabh Jain Pharma |


